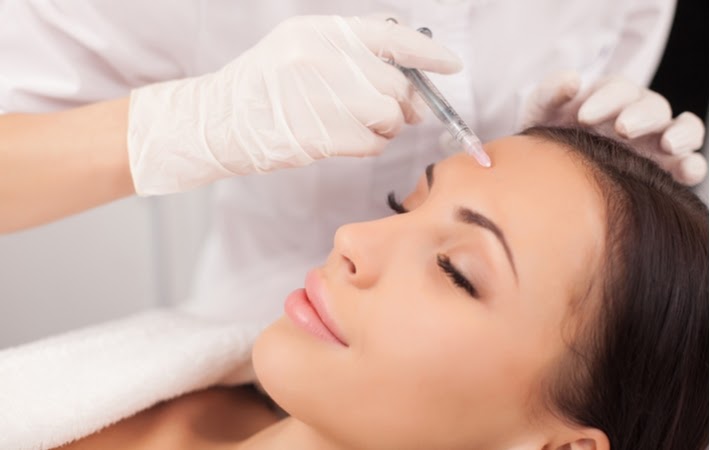
Botox has become one of the most widely recognized cosmetic treatments worldwide, but many people remain curious about its actual composition. This neurotoxin-based treatment has medical and cosmetic applications, yet its origins and manufacturing process remain mysterious to most patients. Understanding what Botox contains and how it works can help you make informed decisions about potential treatments.
Understanding Botulinum Toxin Type A
Botox contains botulinum toxin type A as its active ingredient. This neurotoxin comes from the bacterium Clostridium botulinum, a naturally occurring organism found in soil, lakes, forests, and the intestinal tracts of mammals and fish. The bacteria produce several types of botulinum toxin, but type A is the specific variant used in Botox formulations.
Scientists isolate and purify this toxin through a complex laboratory process. The purification removes harmful bacteria while preserving the therapeutic properties of the toxin. This process transforms a potentially dangerous substance into a controlled medical treatment. The botulinum toxin works by blocking nerve signals to muscles, causing temporary muscle relaxation. This mechanism makes it effective for both cosmetic applications, such as reducing wrinkles, and medical treatments, including muscle spasticity and chronic migraines.
Exploring Components and Formulation
Botox contains several inactive ingredients that stabilize the active toxin and make injection possible. These additional components include human albumin, sodium chloride, and sucrose. Human albumin serves as a stabilizing protein that prevents the botulinum toxin from degrading during storage and transport. This blood-derived protein helps maintain the potency and effectiveness of the treatment over time. The albumin undergoes extensive testing and purification to meet safety standards.
Sodium chloride, commonly known as salt, helps maintain the proper pH balance and osmotic pressure of the solution. This component makes the injection more comfortable and reduces tissue irritation at the injection site. Sucrose, a form of sugar, acts as another stabilizing agent. It helps preserve the molecular structure of the botulinum toxin and prevents protein aggregation that could reduce treatment effectiveness.
Identifying Manufacturing and Safety Standards
The production of Botox follows strict pharmaceutical manufacturing standards. The process begins with controlled bacterial cultivation in sterile laboratory conditions, where manufacturers grow Clostridium botulinum cultures and harvest the toxin they produce. Following harvesting, the toxin undergoes multiple purification steps. These processes remove bacterial remnants, unwanted proteins, and other contaminants.
Quality control testing occurs at multiple stages throughout manufacturing. Each batch undergoes potency testing, sterility testing, and safety evaluations before receiving approval for distribution. This rigorous testing process helps maintain consistent product quality and patient safety. The final product comes as a freeze-dried powder that medical professionals reconstitute with saline solution before injection. This freeze-drying process, called lyophilization, extends shelf life and maintains product stability.
Learn More About Botox
Botox contains botulinum toxin type A as its active ingredient, derived from purified bacterial cultures. The formulation includes stabilizing components such as human albumin, sodium chloride, and sucrose that maintain product effectiveness and safety. Understanding these components can help you have informed discussions with medical professionals about Botox treatments. The complex science behind this simple injection demonstrates the extensive research and development that makes modern cosmetic and medical treatments possible.